Update on Claim Interpretation from the Courts
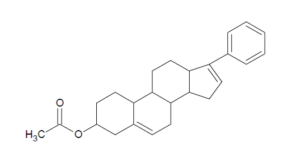 In another precedential opinion, the Federal Circuit Court of Appeals continued its focus on defining claim term meanings in light of the specification and prosecution history.[i] At issue was Auerbach, et al. (U.S. Pat. 8,822,438), which involves the use of a CYP17 enzyme inhibitor with an anticancer or steroid to treat prostate or breast cancer.[ii] Notably, the patent provides using the anti-androgen abitraterone acetate, seen below, and prednisone.[iii]
In another precedential opinion, the Federal Circuit Court of Appeals continued its focus on defining claim term meanings in light of the specification and prosecution history.[i] At issue was Auerbach, et al. (U.S. Pat. 8,822,438), which involves the use of a CYP17 enzyme inhibitor with an anticancer or steroid to treat prostate or breast cancer.[ii] Notably, the patent provides using the anti-androgen abitraterone acetate, seen below, and prednisone.[iii]
Abitraterone acetate is an oral anti-androgen used in prostate cancer treatment, designed to inhibit enzymes involved in androgen steroid synthesis.[iv] The patent was asserted against Amneal Pharmaceuticals LLC, Dr. Reddy’s Labs, Inc., Wockhardt Bio AG, Mylan Pharmaceuticals Inc., and more.[v] Pat. ‘438 was challenged, via Inter Partes Review and through litigation, as obvious. Read More


 The Federal Circuit has been active in clarifying what constitutes patentable subject matter in the medicinal and biotechnology fields, issuing a precedential and nonprecedential opinions over the last few weeks. The cases highlight the importance of including manipulation steps in patent claims, i.e. where a human provides an active step in the claimed subject matter that is not readily known.
The Federal Circuit has been active in clarifying what constitutes patentable subject matter in the medicinal and biotechnology fields, issuing a precedential and nonprecedential opinions over the last few weeks. The cases highlight the importance of including manipulation steps in patent claims, i.e. where a human provides an active step in the claimed subject matter that is not readily known.
 The U.S. Patent and Trademark Office recently issued new guidelines to determine patent eligibility. For years after Alice,
The U.S. Patent and Trademark Office recently issued new guidelines to determine patent eligibility. For years after Alice, The Federal Circuit recently issued a much needed development in patentability case law, in Vanda Pharma. Inc. v. West-Ward Pharma. Int’l Ltd.
The Federal Circuit recently issued a much needed development in patentability case law, in Vanda Pharma. Inc. v. West-Ward Pharma. Int’l Ltd.